CMS Releases Meaningful Use Changes in Interim Final Rule with Comment CMS has announced the release of an Interim Final Rule with Comment (“IFC”) that makes several changes to the Medicare and Medicaid EHR Incentive Programs and 2014 EHR Certification Criteria. Short and sweet, the...
read more
OIG Finds Fault with CMS Meaningful Use Oversight
OIG Finds Fault with CMS Meaningful Use Oversight In a report released on November 29, the Office of Inspector General (OIG) chastised CMS for not doing a better job of pre and post-payment oversight for the Medicare and Medicaid EHR Incentive Programs (Meaningful Use). As of September...
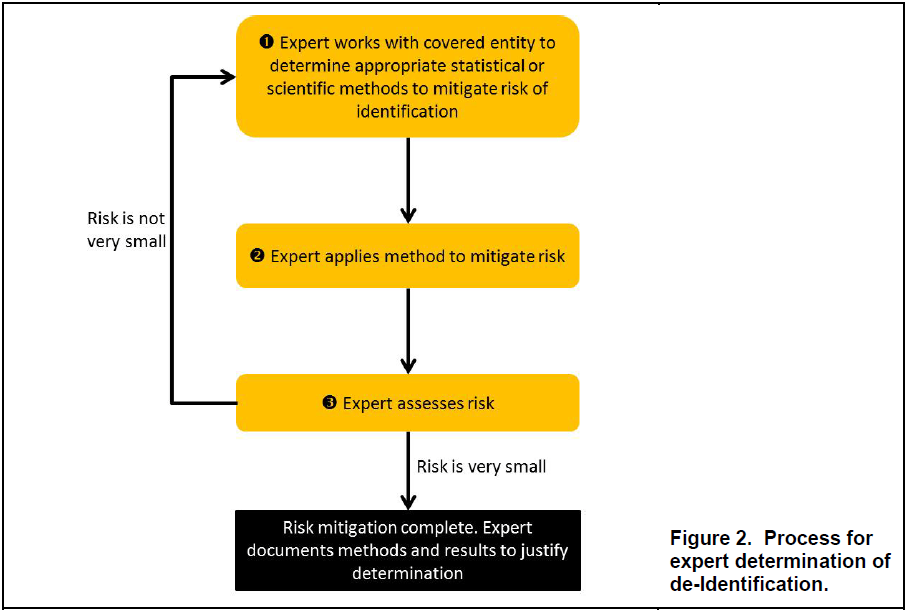
OCR Releases HIPAA De-identification Q&A Guidance
OCR Releases HIPAA De-identification Q&A Guidance With the weekend coming up, why not take a break from the holiday frenzy and read through OCR’s new HIPAA De-identification guidance. The approximately 30-page guidance document is an easy read, even for those of us who aren’t...